Distilling Other Things Besides Essential Oils
Distilling is an ancient art.
The process of distillation was first brought to us through the ancient practice of Alchemy.
Distillation is a method for separating the "Essence" of the plant from the corporeal mass of the plant. This is the isolation and separation of essential oils from the body of the plant.
In Alchemical texts, the essential oil is referred to as the "Sulfur" This is the soul, or spirit of the plant. The idea for the Alchemists was (and still is) the separation of the components of every material (plant and mineral) and the recombination of those components to make something that hasn't existed before.
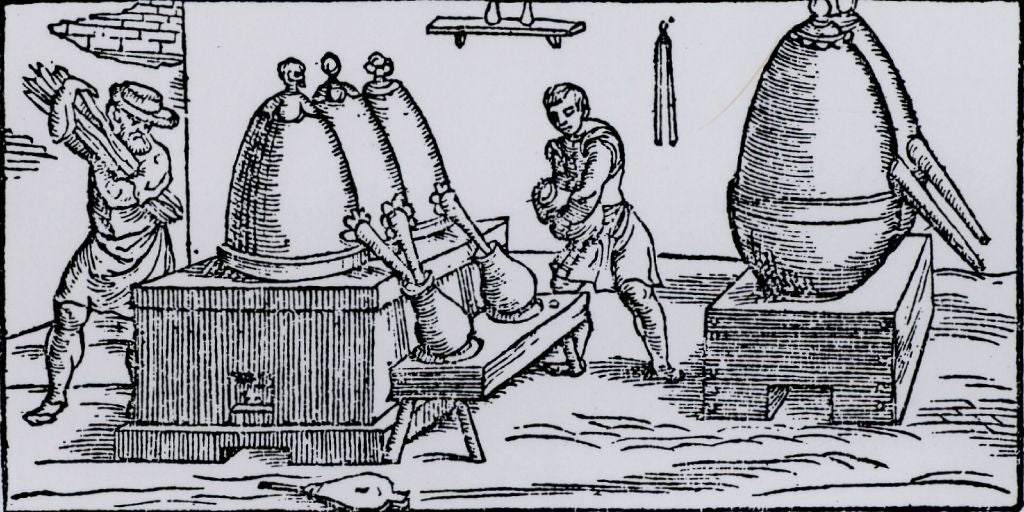
Through invasion and trade, distillation apparatus found its way to Europe from the Middle East.
Alchemy was also practiced in Europe. The Europeans developed other uses for this valuable distillation equipment through their Alchemical experimentation. The production of alcohol through the distillation fermented grains. Alcohol became valuable for making tinctures, medicinal extracts, perfumes and consumable beverages.
Farmers in Europe grew wheat and rye. Wheat and rye are used for making bread, a dietary staple. A problem facing the farmers, was the storage of the grain products which they did not use, sell or trade. A discovered solution, was the fermentation of excess grains and the "spirits" resulting from their distillation.
The process of fermentation yields two products; carbon dioxide and alcohol. Alcohol is separated from the fermented product (grains and fruits) through the use of distillation.
Fermentation is an enzymatic action caused by microorganisms that enter a mixture, either by addition or through the exposure to the atmosphere. This is how dough is made for making bread. Water is added to flour, yeast is added and the mixture is allowed to sit. Fermentation begins during the "sitting" process. The bubbles that you see in bread dough are pockets of carbon dioxide. If you permit your mixture to sit for a long period of time, you will see the creation of alcohol in the mixture.
The separation of liquids from fermented solids is possible by distilling the fermented mass. Alcohol is volatile and readily separates at relatively low temperatures from the mass.
Water boils at 100º C or 212ºF. Ethanol (the alcohol produced through fermenting grains) boils at 78.3º C or 172.94º F. The carbon dioxide in fermented grains evaporates into the atmosphere. This leaves behind the solid mass of fermented grain, water and alcohol.

During distillation the fermented mixture is boiled. The steam resulting from boiling carries water and alcohol to the condenser as steam. Pipes (or tubes) carrying the steam go through cooling waters in the condenser. The cooling process causes the steam to revert to a liquid state containing both water and ethanol. This first distillation will contain approximately 7% of alcohol dissolved in the water.
To separate the alcohol from the water/alcohol mixture a second distillation is necessary. At this time there is no longer a solid fermented mass to deal with but rather a liquid that needs to be divided into components. This separation in the second distillation process employs the use of temperature to separate the liquid components by boiling point differential.

Wine can be distilled for making Eau de Vie and Brandy. Fermented grains are the basis for whiskey and other distilled spirits. Rum is from fermented sugar cane. Aging the distilled spirits in oak barrels adds another nuance to the flavor of the distilled spirits.


